Authored by Ethan Carter (Regional Crop IPM Agent) and Pratap Devkota (UF/IFAS Weed Specialist)
Every applicator can think of an example where their pesticide (herbicide, insecticide, fungicide) provided less than desirable control. What you may not realize is that spray water quality factors play a critical role in pesticide efficacy. The pH, dissolved minerals, suspended solids, and temperature can all impact pesticide performance.
A pH of 7 indicates the water concentrations of (H+) and (OH–) are balanced for neutrality, under 7 indicates acidic conditions (higher levels of H+) and over 7 indicates an alkaline or basic pH (higher levels of OH–). In most cases, herbicides, insecticides, and fungicides tend to perform best in slightly acidic water with a pH range of 4 to 6.5; however, there are several pesticides which perform better at alkaline pH.
| Interpretation/Categories | Hardness (ppm) | Hardness (grain) |
| Soft | 0 – 17 | 0 – 1 |
| Relatively soft | 17 – 50 | 1 – 3 |
| Moderately hard | 50 – 120 | 3 – 7 |
| Hard | 120 – 170 | 7 – 10 |
| Very hard | >170 | >10 |
UF/IFAS Soil and Water Lab Hardness Scale: 1 grain = 17 ppm
In the Panhandle, 25 water samples were collected from Escambia to Jackson counties and tested to determine pH and hardness. There was a huge variation in water pH and hardness among the samples collected. In general, water samples collected from Escambia to Okaloosa counties were found to be in the acidic range (pH of 4.57 to 7.14) with hardness levels between soft and relatively soft (17 to 68 ppm). Whereas, water samples collected from Walton to Jackson counties were found to be higher in pH (pH of 7.5 to 8.2) with hardness levels between hard and very hard (137 to 222 ppm). The general understanding is that basic pH can be good for some herbicide products (but not for all) within group 2 – ALS inhibitors and group 14 – PPO inhibitors. Other herbicides like RoundUp, 2,4-D, and Liberty tend to perform better at acidic pH ranges. Water hardness is a frequent issue, and there are different conditioner options available for use based on a specific product’s label. It is important to read the product label because some pesticide labels forbid the use of ammonium sulfate (like the newer dicamba formulations used in cotton and soybean) or other conditioners.
It is advisable to get the well or other water source used for spray tanks tested for factors that may influence pesticide applications. If you are interested in having your water tested,use the following link for the UF/IFAS Water Test Form. This form also includes the basic instructions for submitting a sample.
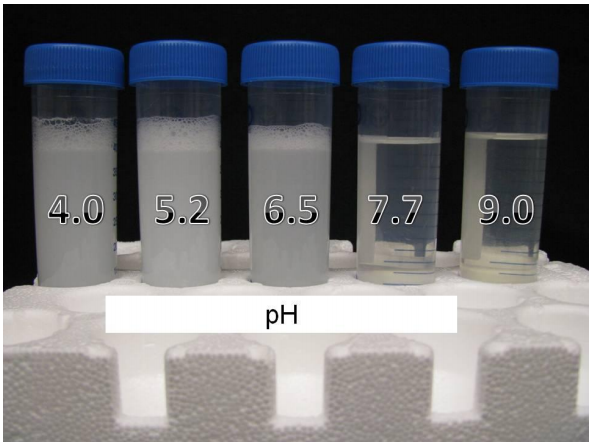
Effect of water pH on solubility of Sharpen herbicide. At acidic water herbicide has a solubility issue (forms precipitate), but at basic pH there is no issue (forms clear solution). Source: Roskamp et al. (2013).
Insecticide families such as the carbamates and organophosphates most commonly have breakdown problems in alkaline water, undergoing the chemical reaction known as alkaline hydrolysis. The speed and intensity of this breakdown are dependent on the specific chemical properties of the pesticide, water pH, and length of time it remains in the spray tank after mixing. At higher pH values between 8 and 9, their control can be greatly reduced or completely lost due to rapid breakdown.
Herbicides such as 2,4-D, dicamba, and glyphosate are weak acids, meaning that they have an overall negative charge after they are mixed with in water. The negative charge can easily bind with the positive charge of hard water minerals/cations and form a precipitate. This prevents herbicide from entering into the plant, and results in reduced herbicide effectiveness (less weed control) when applied with water containing high levels of dissolved minerals (hard water cations such as calcium, magnesium, iron, zinc etc.).
Suspended solids found in murky, or muddy water include silt, clay, and other organic matter. These particles can bind herbicide molecules and settle to the bottom of the tank. Cation exchange and physical compatibility lead to the binding of pesticide molecules with these particles instead of functioning properly. This can be a serious issue when drawing water directly from a pond or canal.
A water temperature study at Purdue University found that mixing certain herbicides with cold water (around 41◦F) or very hot water (around 132◦F) can also impact their performance on specific weeds. This should be kept in mind for water stored in above ground tanks for few days, as the temperature needs to be monitored.
One or more of these spray water variables can result in reduced solubility, decreased absorption, and decreased half-life of many pesticides. Half-life is the time it takes for one half of the amount of pesticide active molecule to breakdown or degrade. The half-life of a pesticide can be a matter of minutes, hours, or days. For example, flumioxazin (active ingredient in Valor or similar products) will persist in water for several days at pH 5, but has a half-life of 24 hours at pH 7, and only 15 minutes at pH 9. A table listing the optimum pH and half-life of multiple pesticides (insecticides, fungicides, and insecticides) can be found in an article titled Effect of Water pH on the Stability of Pesticides by Michigan State University Extension (and others cited below).
For more information regarding water pH and pesticide performance, see the following articles:
- Water pH and the Effectiveness of Pesticides
- pH and Water Modifications to Improve Pesticide Performance
- Pesticide Performance and water Quality
- The pH of the Spray Water is Very Important
For questions or more information, contact your local extension office.
- 2024 Panhandle Row Crop Short Course Highlights & Presentation Links - March 8, 2024
- 2024 Wiregrass Cover Crop Field Day – Rescheduled – March 29 - February 16, 2024
- Panhandle Row Crop Short Course – March 7 - February 9, 2024
