Dr. João Bittar, Dept. of Large Animal Clinical Sciences, College of Veterinary Medicine, & Dr. Juan M. Campos Krauer, Dept. of Wildlife Ecology and Conservation. Cervidae Health Research Initiative. University of Florida
Having healthy animals is the wish of every farmer (livestock and even deer); however, keeping animals healthy requires a good herd health management plan. All animals have various defense mechanisms to prevent or deal with infections. The defense mechanisms of the animal can be directly influenced by age, nutrition, or inappropriate management practices. Stress due to heat, weaning, malnutrition, infection, transport, and other factors can impact how the immune system reacts to a pathogen attack.
An important component of any herd health plan is a vaccination protocol, yet, few vaccines are explicitly developed for deer in the deer industry. Instead, vaccines developed for other species such as cattle, equine, sheep, or goats are used if they have been labeled for use in deer. Still, few studies have measured the efficacy of vaccines in deer; for most vaccines, only anecdotal data are available.
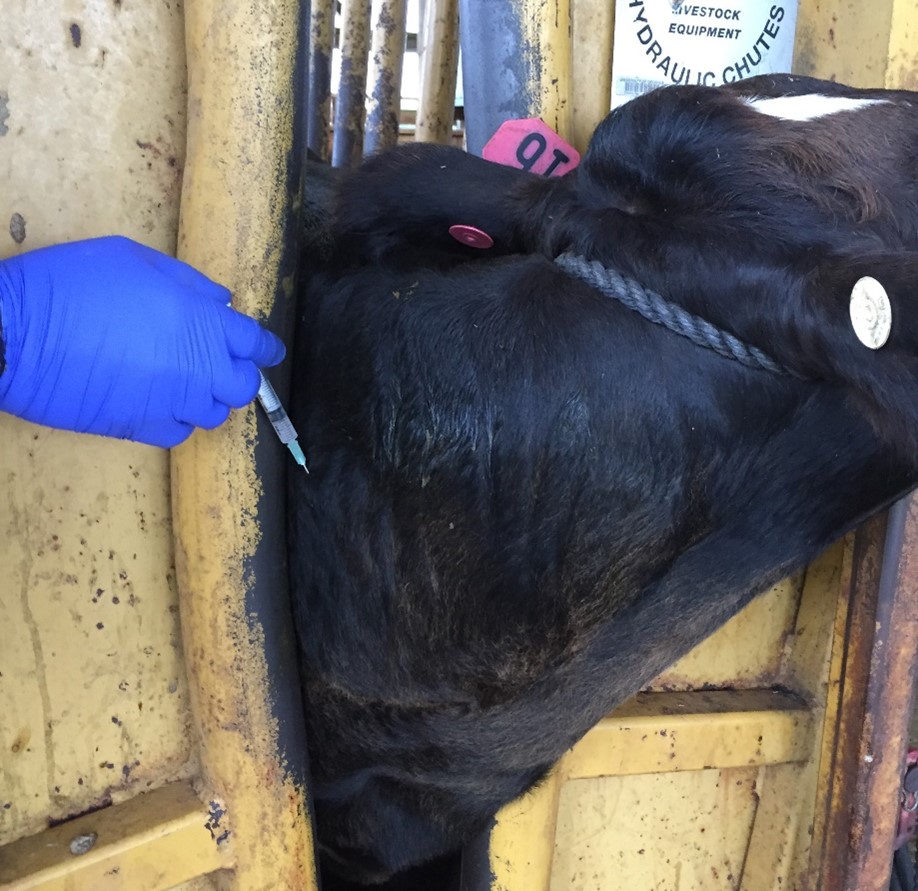
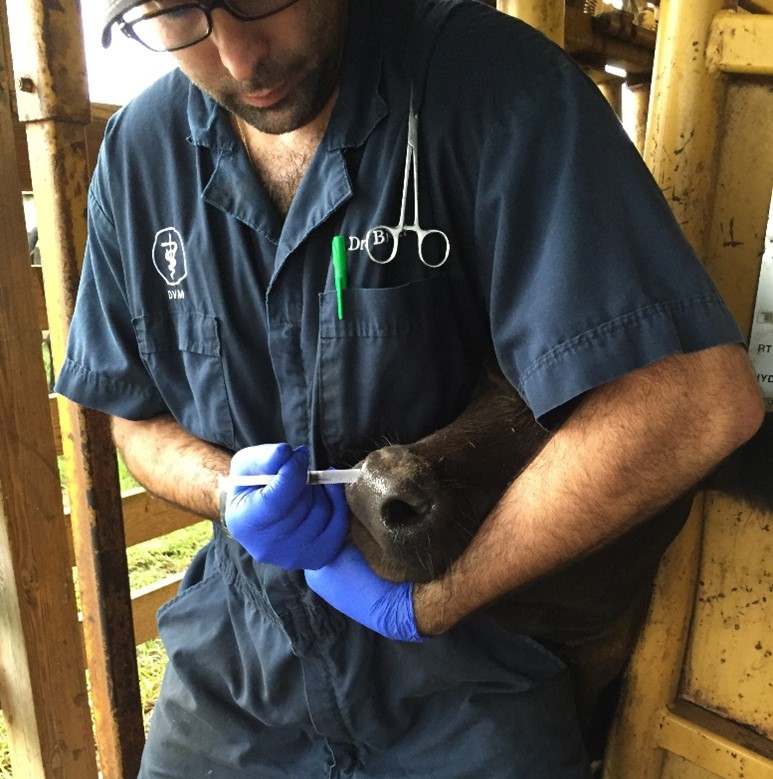
Figure 1: Administration of vaccine subcutaneously (SQ) in a naïve calf using a disposable single-dose syringe. Credit: Dr. João Bittar, UF/IFAS
Most vaccines induce protection by priming the system to mount an antibody response. When an animal has not been exposed to a specific pathogen or comes in contact for the first time, it can be slow to develop antibodies. Most vaccines work by introducing the body’s system to pathogen-specific proteins. Often, without this “sneak preview” of the vaccine, the animal cannot generate an immune response quick enough to clear or destroy the pathogen, making the animal susceptible to the agent and becoming sick. However, if the animal has a robust immune system or has been vaccinated, it will suppress the pathogen and, in time, clear or significantly reduce the infection.
When an animal recovers from disease or has been vaccinated (Figure 1), specific cells from the immune system will acquire the ability to remember and recognize the pathogens (virus, bacteria, toxin, or parasite) or parts of the pathogen known as antigens. The next time the immune system recognizes these antigens, it will immediately trigger the production of specific antibodies by specialized cells, which will work to destroy the pathogen.
–
How do vaccines work
Vaccines expose the animal to parts of pathogens, challenging the immune system to react to a possible pathogen invasion by creating memory cells for the antigens belonging to that specific pathogen. In the future, if the animal is exposed to the same pathogen, the immune system will quickly generate a response before the pathogen can cause disease. Each antibody is usually specific for only one antigen. Because of this, the immune system keeps a supply of millions of different antibodies on hand to be prepared to overcome any foreign invader. For a naïve animal (an animal that never was exposed to the pathogen), it may take 7 to 14 days after exposure to an infectious agent for the body to develop immunity to an antigen, which is plenty of time for some pathogens to wreak havoc on the body. On the other hand, it often takes only 48 hours to mount an immune response to the same antigen in a vaccinated animal.
There are several types of vaccines used in humans and animals. The majority of the licensed veterinary vaccines currently in use are inactivated (killed) vaccines, live-attenuated vaccines, or toxoids. All these represent different strategies used to reduce the risk of illness while retaining the ability to induce a beneficial immune response.
–
Types of vaccines
- Attenuated vaccine: Some vaccines contain live, but alterted microorganisms. Many of these are active viruses cultivated under conditions that disable their virulent properties or use closely related but less dangerous organisms to produce a broad immune response. Although most attenuated vaccines are viral, some are bacterial. Attenuated vaccines have some advantages and disadvantages. Attenuated, or live, weakened, vaccines typically provoke more durable immunological responses. But they may not be safe for use in immunocompromised individuals.
– - Inactivated vaccine: Some vaccines contain inactivated but previously virulent microorganisms that have been destroyed with chemicals, heat, or radiation. They are considered an intermediate phase between the inactivated and attenuated vaccines. Examples include IPV (polio vaccine), hepatitis A vaccine, rabies vaccine, and most influenza vaccines.
= - Toxoid vaccine: Toxoids are made from inactivated toxic compounds produced by microorganisms that, when activated, cause damage to cells. Examples of toxoid-based vaccines include tetanus and clostridium. Not all toxoids are for toxins created by microorganisms; for example, Crotalus atrox toxoid is used to vaccinate dogs against rattlesnake bites.
– - Subunit vaccines: These vaccines contain short, specific proteins that are the same as the antigens of the target pathogen. A subunit vaccine uses a component to induce an immune response rather than introducing an inactivated or attenuated microorganism to an immune system.
– - Conjugate vaccine: Certain bacteria have a polysaccharide outer coat, which is a weak antigen. The immune system has a more robust response by linking these outer coats to proteins (toxins), which are strong antigens.
– - Outer membrane vesicles (OMVs): OMVs are released spontaneously during growth by many groups of bacteria. They have the ability to naturally provoke an immune response in the body of a human or other animal and can be manipulated to produce potent vaccines. The best known OMVs vaccines are those developed for serotype B Meningococcal disease.
– - Heterologous vaccines: These are also known as “Jennerian vaccines“. The heterologous vaccines contain pathogens from other animals that either do not cause disease or cause mild illness in the organism being treated. The classic example is Jenner’s use of cowpox to protect against smallpox. A current example is using the vaccine made from Mycobacterium bovis to protect against tuberculosis in humans.
– - Viral vector vaccines: This vaccine uses a nonpathogenic virus to insert pathogen genes in the body to produce specific antigens, such as surface proteins, to stimulate an immune response. The new EHDV vaccine uses this technology in combination with the subunit vaccine technology.
– - RNA vaccine: A mRNA vaccine is a novel type of vaccine composed of nucleic acid RNA, packaged within a unique delivery system like lipid nanoparticles. Several COVID-19 vaccines are RNA vaccines and have received emergency use authorization in some countries. For example, the Pfizer-BioNTech and Moderna mRNA vaccines have emergency use authorization in the US.
–
Vaccination is only one tool to prevent disease and cannot be used as a standalone practice to prevent disease or infection on the farm. Vaccination does not result in immediate immunity or resistance against diseases in all vaccinated animals. It takes time for the animal’s immune system to react to the vaccine. Therefore, if an animal is vaccinated, it does not automatically mean that the animal cannot be infected or develop the disease. The degree of protection is directly dependent on the animal’s health, how well the vaccine is matched to the pathogen, and how well the vaccine is administered to the animal. Vaccines come in many types, but all are delicate organic products that need to be managed and administrated correctly to ensure their effectiveness.
–
Important factors to consider when using vaccines
- Order vaccines from a trusted source. Always order from a trusted veterinary supplier or directly from the company producing the vaccine.
_ - Order an adequate amount of vaccine. Always add an extra 10 percent to your order to account for possible vaccine losses during animal handling. If possible, order bottles with fewer doses. Shelf life varies for each type of vaccine. Some vaccines have hours of efficacy after being mixed, some longer. However, it is not recommended to use an open vaccine bottle kept in the refrigerator after long periods. Again, fewer dose bottles help calculate what you need for that day.
– - Correct storage conditions. Check instructions on how the vaccine should be stored. Most animal vaccines require refrigeration at 35 – 45 ˚F (2 – 7 ˚C). Make sure your storage refrigerator works properly, place a thermometer in a prominent place so you can check the temperature often. Refrigerators held in barns or open sheds can have temperature variations throughout the day, affecting the temperature. Never freeze a vaccine, nor let it get too warm. Always avoid direct sunlight on your vaccine.
– - Expiration dates. Always check expiration dates, and always start by using the oldest first. Once opened, always label the bottle with the date, particularly if you will store it for later use.
– - Correct preparation and vaccine shelf life after mixing. Follow directions on the bottle to ensure its effectiveness. This is very important; some vaccines need to be reconstituted with sterile water or have components that need to be mixed. Make sure you follow the instructions and always mix gently. Remember, vaccines are delicate organic products. Prevent temperature shocks. Grabbing a cold vaccine bottle with warm hands can rapidly change the container’s temperature and affect its efficacy.
– - Exposure to UV light. Do not expose vaccines to Ultraviolet light from the sun. Some vaccines can be rapidly deactivated if exposed to UV light.–
-
Proper injection techniques. Always inject the vaccine according to the manufacturer’s directions. In animals, most vaccines are injected either under the skin – subcutaneous (SQ), in the muscle – intramuscular (IM), squirted in the nasal cavity – intranasal (IN) (Picture 2), or directly in the blood stream – intravenous (IV). Using the correct technique and location according to the species is essential. Use the right needle size and avoid reusing the same needle on another animal if possible, to reduce the risk of some disease transmission. Darts can be used for intramuscular (IM) injections, even though it is not ideal. When using a dart, there are many variables that you need to consider. Common mistakes include missing, hitting the wrong spot, darting the same animal twice, or an incomplete discharge of the dose. If you are not sure if the animal received the full dose, a second full dose is recommended. There are some great guidelines regarding vaccine management and administration in the livestock industry presented by the Beef Quality Assurance (BQA) program. These best management practices for vaccines can also be applied to other species and aim for successful vaccination outcomes allied to food safety.
– - Good records. Always record dates, animal ID, and vaccine lot numbers. Keeping good records is critical to improving herd health over time and may be critical for importing or exporting animals.
– - Correct disposal of vaccine containers. Some vaccines have products that need special disposal, so you don’t want to keep them on your farm. Read the instruction for proper disposal of used containers. Regulations can vary by state.
_
- Emergency information. In case of an accidental human injection or exposure to the vaccine, it is always important to have emergency numbers at hand for everyone working on the farm.
–
In conclusion, this article provides a basic guide that can help you to better understand the basic mechanism and types of vaccines available, as well as some basic best practices of vaccine management. This information can help you be successful in your next herd vaccination to guard the health of your herd. In the US, animal vaccines are regulated by the United States Department of Agriculture (USDA), and further information about licensed vaccines can be found on their website:
USDA Animal Health – Veterinary Biologics
–
References
- COVID-19 and Your Health. Centers for Disease Control and Prevention.
- Different Types of Vaccines – History of Vaccines
- Leo van der Pol, Michiel Stork and Peter van der Ley. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015 Sep; 10(11): 1689–1706. doi: 10.1002/biot.201400395
- McVey and Shi, 2010 S. McVey, J. Shi. Vaccines in veterinary medicine: A brief review of history and technology. Veterinary Clinics of North America – Small Animal Practice, 40 (3) (2010), pp. 381-392, 10.1016/j.cvsm.2010.02.001
- Meeusen et al., 2007 E.N.T. Meeusen, J. Walker, A. Peters, P.P. Pastoret, G. Jungersen. Current status of veterinary vaccines. Clinical Microbiology Reviews, 20 (3) (2007), pp. 489-510, 10.1128/CMR.00005-07
- National Cattlemen’s Beef Association. Beef Quality Assurance: BQA_Manual_Final. Accessed on Oct. 21, 2021. Centennial, CO. https://www.bqa.org/Media/BQA/Docs/bqa_manual_final.pdf
- Sinha JK, Bhattacharya S. A Textbook of Immunology. Academic Publishers. p. 318. ISBN 978-81-89781-09-5.
- Polysaccharide Protein Conjugate Vaccines. www.globalhealthprimer.emory.edu.
- Pollard AJ, Bijker EM (2020-12-22). A guide to vaccinology: from basic principles to new developments. Nature Reviews Immunology. 21 (2): 83–100. doi:10.1038/s41577-020-00479
- Animal and Plant Health Inspection Service/ Animal Health/Biologics
- Vaccine Types. Vaccines.org. Office of Infectious Disease of the United States Department of Health and Human Services.
- Join Our Cow-Calf Parasite & Infectious Diseases Study for Free! - January 12, 2024
- Animal Vaccines – Principles, Types, and Important Points to Know - June 17, 2022