
Farmers utilizing urea for nitrogen (N) fertilization should be aware of climatic conditions that can reduce crop yields. Frequent and intense rainfall events cause N leaching through the root zone, and high soil temperatures, low soil moisture, high soil pH, and low soil nutrient holding capacity cause N volatilization into the atmosphere. Credit: Doug Mayo, UF/IFAS.
Arun Jani and Mike Mulvaney, West Florida Research and Education Center
Climatic conditions in North Florida can pose serious challenges to growers aiming to maximize returns on investments made in nitrogen (N) fertilizer. Most plant available N in local soils is in the nitrate (NO3–) form. Frequent and intense rainfall events, coupled with the lighter-textured soils that dominate much of North Florida, can lead to substantial NO3– leaching. Additionally, local climatic conditions often favor gaseous losses of N from surface-applied urea through ammonia (NH3) volatilization. Conditions favoring volatilization include high soil temperatures, low soil moisture, high soil pH, and low soil nutrient holding capacity. Although summer rainfall can be plentiful, rapid drainage on lighter-textured soils, in addition to dry spells at N topdressing, can create low moisture conditions at the soil surface. The possibility also exists for soil microsites high in pH that allow for volatile losses of NH3.
Given how susceptible N fertilizer is to environmental losses in North Florida, UF/IFAS Extension recommends that growers follow the “4Rs” of nutrient management as a best management practice to optimize N fertilizer recovery by crops. These four fertilizer management principles are:
- Right source of fertilizer – Think of expense, ease of application, and soil health.
- Right rate of fertilizer– Consult your soil test report and think about yield goals.
- Right placement of fertilizer – Banding vs. broadcasting, depends on crop in question.
- Right timing of fertilizer – When does rapid nutrient uptake occur for your crop?
A UF/IFAS Extension publication describing each factor in detail and discussing how soil test reports can be used to help growers optimize returns on their fertilizer investments is available online: The Four Rs of Fertilizer Management. In addition to the 4Rs, there are commercially available compounds known as nitrification and urease inhibitors that may be applied at N fertilization to reduce the risk of N losses to the environment.
Nitrification and Urease Inhibitors
Nitrification inhibitors are compounds that slow down the activity of soil microbes that are responsible for nitrate formation, which may reduce the risk of large scale NO3– leaching under intense rainfall conditions. The most commonly available nitrification inhibitors are 2-chloro-6-(trichloromethyl)-pyridine (nitrapyrin) and dicycandiamide (DCD). Both of these compounds are most effective on light-textured, low organic matter soils, like those found in much of North Florida. However, field experiments have shown that nitrapyrin and DCD do not consistently improve crop yields in North Florida. In Gainesville and Hastings, Florida, these compounds only improved potato yields in one out of five field studies (Martin et al., 1993). In this same region, application of urea plus DCD only increased sweet corn yields at low N rates that would not be profitable to use (Frye et al., 1989). The researchers who led these experiments concluded that nitrification inhibitors should not be recommended in the region.
Urease inhibitors have a different mode of action than nitrification inhibitors, but serve the same purpose of reducing N environmental losses. These compounds work by slowing down the rate at which urea is converted to NH3 by reducing urease enzyme activity in the soil. Urease inhibitors can be mixed with or coated onto urea-containing fertilizers, and should not be confused with polymer-coated urea products in which N release is temperature-dependent. N-(n-Butyl) thiophosphoric triamide (NBPT) is the most commonly used urease inhibitor. This compound can reduce urease activity for 7 to 14 days, after which rain, irrigation, or soil incorporation would be needed to limit ammonia losses (Figure 1). While field experiments investigating crop yield response to urease inhibitors in the Southeast are lacking, research has shown that NBPT-treated urea can reduce ammonia losses by 52% when compared to untreated urea (Silva et al., 2017). Therefore, urease inhibitors, namely NBPT, are a sound investment in nutrient management programs in North Florida.
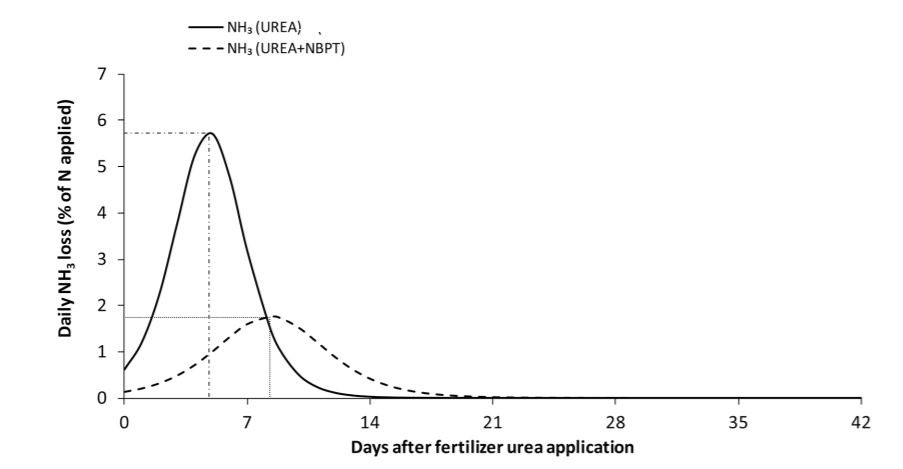
Figure 1: Daily NH3 losses during 42 days after application for urea and urea treated with NBPT. Estimates are based on data collected from 35 different studies around the world (source: Silva et al., 2017).
Take Home Points
- Climatic conditions in North Florida are highly conducive to N fertilizer losses to the environment through leaching and gaseous emissions (volatilization).
- Following the 4Rs is a best management practice that can improve N fertilizer recovery by crops.
- Use of nitrification inhibitors are not recommended, because field experiments indicate they do not have a consistent positive effect on crop yields.
- Urease inhibitors can reduce NH3 losses from urea, are relatively inexpensive, and can be viewed as an insurance policy protecting against NH3 volatilization.
References
Frye WW, Graetz DA, Locascio SJ, Reeves DW, Touchton JT. Dicyandiamide as a nitrification inhibitor in crop production in the southeastern USA. Communications in soil science and plant analysis. 1989 Dec 1;20(19-20):1969-99.
Martin HW, Graetz DA, Locascio SJ, Hensel DR. Nitrification inhibitor influences on potato. Agronomy journal. 1993;85(3):651-5.
Silva AG, Sequeira CH, Sermarini RA, Otto R. Urease inhibitor NBPT on ammonia volatilization and crop productivity: a meta-analysis. Agronomy Journal. 2017;109(1):1-3.
- Four Early-Season Lessons from 2020 Peanut Production - April 9, 2021
- Sprayer Calibration Tables – Calibration Made Easy - October 30, 2020
- Stand Issues – Should You Replant Your Peanut Field? - May 15, 2020
