Monalisa Lima, Ameer Megahed, Alireza Shahraki, João Bittar, DVM, MSc., PhD., UF Large Animal Clinical Sciences, College of Veterinary Medicine, and Lauren Butler, UF/IFAS Extension Okeechobee County
Introduction

Figure 1: Pond on a private ranch located in Okeechobee County, FL, featuring red algae, Karenia brevis on its surface. Credits: Lauren Butler, UF/IFAS
The rapid proliferation of certain toxic microorganisms in fresh, brackish, marshy regions, low-salinity estuaries, or marine waters can negatively impact humans, animals, and the ecosystem. This phenomenon, which can persist for weeks or months, is referred to as Harmful Algal Blooms (HAB) and originates from the abundant and disordered growth of algae, cyanobacteria, dinoflagellates, and diatoms (Carmichael, 1994). They sometimes look like foam, scum, or a tiny mat on the surface of water (Wilson et al, 2018). They can even make water appear in different colors, including green, blue, red (Figure 1), pink, and brown (Carmichael 1994). Genera of most interest on the coast and inland of the state of Florida are Euglena, Microcystis, Aphanizomenon, and Karenia, popularly known as Red Tide (Lackey & Phelps, 1964; Landsberg et al., 2009; Hall-Scharf & Ubeda, 2019).
The impacts of harmful algal blooms (HAB) are variable (Hall-Scharf, 2009; Bischoff, 2023). Even when toxins are not produced, intense blooms can significantly reduce water quality, leading to decreased water intake by cattle. Since water is an essential nutrient that directly affects cattle performance and health, stakeholders in the cattle industry must prioritize a consistent supply of high-quality water. Poor water quality or scarcity can result in lower feed intake, reduced weight gains, decreased milk production, and overall negative health outcomes in cattle (Willms et al., 2002).
–
Causes
It is generally accepted that eutrophication is the driving factor that leads to HAB. Eutrophication is characterized by an influx of a limiting nutrient(s) resulting in increased growth of algae and/or other microorganisms (Yang et al., 2008). The influx of these nutrients can come from various sources. Contamination of aquatic ecosystems by organic matter and the incorrect application of fertilizers, runoff from animal confinement areas, rainwater from urban and suburban areas, and discharges from wastewater treatment plants can all contribute to the eutrophication process. Eutrophication generates intense biological growth. This excessive growth results in the depletion of oxygen in the water, as decomposing algal biomass consumes oxygen, leading to hypoxic conditions that can be detrimental to aquatic life. (Figure 2) (Lackey & Phelps, 1964; Landsberg et al., 2009; Wilson et al., 2018).
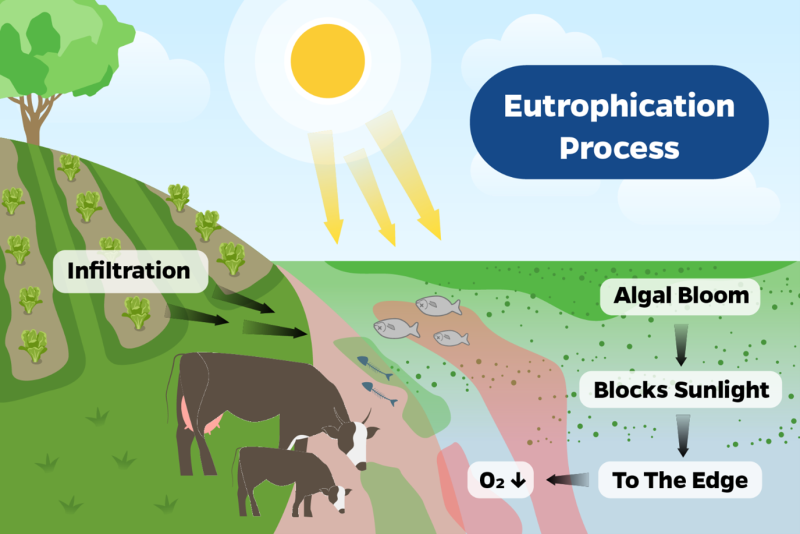
Figure 2: Esquematizacion Eutrophication Process. An increase in nutrient levels in the water is observed, especially on warmer days, as a result of the stimulation of photosynthesis and the proliferation of algae and cyanobacteria. This leads to cloudiness of the water, promotes the death of algae and plants that end up releasing toxins, poisoning the water.
–
Weather conditions also play a role in onset of HAB. Winds are useful for homogenizing the microorganisms throughout the water body, which can minimize dense blooms, preventing HAB. However, following storms and hurricanes, the imbalance of the water body and the increased water turbulence and nutrient runoff can contribute to these blooms (Moore et al.,2008). Hot, sunny days and prolonged droughts lead to increased water evaporation, which, combined with organic matter and excessive nutrient levels, accelerates eutrophication, promoting HABs (Meehan & Dakota, 2021). When climatic and nutritional conditions are favorable, multiplication occurs rapidly, with populations doubling in a day or less, and persisting for several weeks, taking on the appearance of foam or a fine mat. Although the onset, intensity, and duration are unpredictable, historically in Florida, harmful algal blooms (HABs) are more frequently observed in late summer and early fall (Landsberg et al., 2009).
Impact to Animals
The presence and intensity of clinical signs differ among individuals according to; 1) their susceptibility, 2) the type of toxin produced by the algae, 3) the amount of toxin ingested by the animals and 4) how animals are managed (Orr et al., 2003). The animals most likely to be affected are those that tend to drink water from the edge of the waterbody, like calves, as they do not enter beyond the toxic margin (Meehan & Dakota, 2021).
From the perspective of livestock health and welfare, the most important toxins produced by HAB are neurotoxins, hepatotoxins and dermatoxins. Neurotoxins affect the nervous system, resulting in clinical signs such as restlessness, muscle tremors, resistance to walking, weakness, motor incoordination, sialorrhea, respiratory distress, seizures and coma (Carmichael, 1994). If any animal exhibits symptoms of neurotoxicity they should be immediately washed, placed in a shaded area, and a veterinarian should be contacted as soon as possible. Hepatotoxins affect the liver, manifesting as gastrointestinal signs that include reduced or cessation of ruminal movements, jaundice, watery or bloody diarrhea, dry feces and, to a lesser extent, bluish or whitish mucous membranes (Bischoff, 2023; Orr et al., 2003; Silva et al., 2014). In animals that are lactating, milk production suddenly stops. Animals that are affected by toxin ingestion of dermatoxins (Sharp et al., 2009) will seek shaded places because they can develop manifestations of photosensitization, in the form of edema, erythema, open wounds, urticaria, apathy, and extremely hard, dry skin in depigmented and hairless areas such as the snout, around the eyes, hocks, vulva, foreskin, ear tips in addition to significant weight loss (Silva et al., 2014).
The presence of dead animals near ponds or water reservoirs can be a strong indicator of HAB poisoning (Newcomer, 2022). However, depending on the amount and type of toxin, death can occur within 4-24 hours of ingestion. So far, the main toxins found in coastal and inland Florida include anatoxins, microcystins, euglenophysin, brevetoxins, guanitoxin, cylindrospermopsin, aetokyhonotoxin, cigatoxin, okadaic acid, domoic acid and saxitoxin, from various microalgae such as Microcystis aeruginosa, K. brevis, Dynophysis acuminata, Ostreopsis ovata, Alexandrium catanella, Gymnodinium catenatum and Pseudonitzschia spp, among others (Carmichael, 1994; Hall-Scharf and Ubeda, 2019; Larkin and Adams, 2020, Moreira et al., 2022).
–
Prevention
Although intense blooms do not always result in the formation of HAB, it is important to follow some guidelines to decrease blooms, because the development of toxicity is unpredictable and changes in the water are generally undetectable:
- Offer clean and plentiful water to the animals;
- Use gloves, masks, and waterproof bib pants when systematically cleaning water tanks and reservoirs. Bleach should be used or a standard on-farm disinfectant, spray with a high-pressure washer;
- Immediately remove all animals from drinking fountains, tanks, or ponds with altered watercolors, such as opaque, cloudy, blue-green, orange, red, shiny, or brown, with or without foam on the surface;
- Do not offer water from reservoirs with dubious quality and without treatment for a minimum period of 3 months, as toxins are resistant to degradation by sunlight and microbial activity (Rasby & Walz, 2011; Hersom & Crawford; 2020);
- Do not use foam, mud, or any other materials that may be extracted from water as fuel or construction materials, such as bricks. When algae die and dry out, the concentration of biotoxins can increase, posing a greater risk. Additionally, heating water or cooking these organic materials does not neutralize the biotoxins, which remain hazardous even after exposure to high temperatures.
- Livestock-mediated bioturbation can help control algae growth and toxic secondary metabolites in potable water ponds by increasing the number of suspended solids and turbidity, even in nutrient-rich environments with high phosphorus and nitrogen concentrations (Wilson et al., 2018).
- Systematically monitor ponds and water reservoirs.
–
Visual Water Monitoring
Visual water monitoring is an important approach to controlling and monitoring the presence and growth of algae in water. Furthermore, it is a simple practice that can be carried out by owners. Every week, a sample of water is taken from the lagoon using a translucent glass cup. If the water is clear, that is, and you can see through it, it is an indication that the water is safe and that the algae is under control. However, if the water is cloudy or particulate matter is present, steps should be taken to clean the tanks or limit access to the water source and begin treatment as algae will probably start to grow. Regular and systematic monitoring using this strategy allows for rapid detection of changes in water quality and can mitigate a potential HAB. It is especially useful in areas where blooms are relatively common. In South Florida, Lake Okeechobee and surrounding areas, including the St. Lucie and Caloosahatchee rivers, typically experience more harmful algal blooms (HABs) from June to October (Lapointe et al., 2018). Similarly, in Northern Florida, the Apalachicola River in the Panhandle has reported blooms, particularly involving species like Karenia brevis and Pyrodinium bahamense, which can impact both marine life and human health (floridadep.gov, 2024).
–
Algal Bloom Management
Mechanically remove dry or dead blooms from medium or large reservoirs using motorized vehicles such as tractors and backhoe loaders. Be sure to wear gloves, a mask, and a disposable coverall during the process. Contact your local extension agent for assistance in implementing chemical control practices, such as using copper sulfate (CuSO₄) in ponds or troughs to control blue-green algae growth when water temperature is above 60°F. It’s important to consult with your local extension agent or specialist, as products used to treat water are regulated and can be toxic to plants and wildlife.
Reporting and Updates
Whenever there is a change in color or suspicion of water quality, the following resources should be consulted and pertinent information reported accordingly :
- To report Blue-Green Algae Blooms¸ Red Tide or other HAB contact the Florida Department of Environmental Protection or call 855-305-3903 or 866-300-9399;
- To report fish kills or diseased contact Florida Fish and Wildlife Conservation Commission or call 1-800-636-0511;
- To report symptoms from exposure to a HAB or any aquatic toxin to the Florida Poison Information Center or call 1-800-222-1222 to speak to a poison specialist immediately.
–
Conclusion
With the ever-changing climate and continued use of commercial fertilizers, eradicating the causative agents of HAB is not feasible. However, the detrimental impacts to the livestock industry can be mitigated by implementing the guidelines outlined here. Specifically, focusing on reducing bloom levels and frequency requires technical expertise, perseverance for early detection and action, and financial support. While late intervention due to sporadic monitoring practices is better than no intervention, the negative impact on animal welfare and ranch economics will be greater compared to what would be excepted with systematic and regimented monitoring and early interventions. Likewise, proper pasture management combined with methodologies to control water potability are effective strategies in the battle against HAB and will consequently reduce direct damage to livestock.
–
References
Bischoff, K. (2023). Algal Poisoning of Animals-Toxicology. Merck Veterinary Manual.
–
Carmichael, W. W. (1994). The toxins of cyanobacteria. Science Animal. Jan;270(1):78-86.
–
Hall-Scharf, B., Ubeda, A. (2019). How Red Tides Impact Manatees. UF/IFAS.
–
Haman, D.Z.; Clark, G.A.; Pitts, D.J.(2009). Excavated Pond Construction in Florida. IFAS CIR939.
–
Hersom, M., & Crawford, S. (2020). Water Nutrition and Quality Considerations for Cattle. UF/IFAS.
–
James Lackey, by B., & Emeritus Earle, P. B. (1964). A Partial Checklist of Florida Fresh-Water Algae and Protozoa with Reference to McCloud and Cue Lakes.
–
Landsberg, J. H., Flewelling, L. J., & Naar, J. (2009). Karenia brevis red tides, brevetoxins in the food web, and impacts on natural resources: Decadal advancements. Harmful Algae,Vol. 8, Issue 4, pp. 598–607.
–
Lapointe, B. E., Herren, L. W., & Paule, A. (2018). A response to frequently asked questions about the 2018 algae blooms in Lake Okeechobee, the Caloosahatchee, and St. Lucie estuaries. EDIS document SG159. UF/IFAS.
–
Meehan, M. A., & Dakota, N. (2021). Cyanobacteria Poisoning (Blue-green Algae). NDSU Extension V1136.
–
Moore, S.K., Trainer, V.L., Mantua, N.J. (2008). Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ Health 7,Suppl 2, S4.
–
Newcomer, B. W. (2022). Toxicologic insults to the bovine liver. Veterinary Clinics of North America: Food Animal Practice, 38(3), 421-432.
–
Orr, P. T., Jones, G. J., Hunter, R. A., & Berger, K. (2003). Exposure of beef cattle to sub-clinical doses of Microcystis aeruginosa: Toxin bioaccumulation, physiological effects, and human health risk assessment. Toxicon, 41(5), 613–620.
–
Rasby, R. J., & Walz, T. M. (2011). Water requirements for beef cattle (G2060). University of Nebraska–Lincoln Extension.
–
Sharp, K., Arthur, K. E., Gu, L., Ross, C., Harrison, G., Gunasekera, S. P., Meickle, T., Matthew, S., Luesch, H., Thacker, R. W., Sherman, D. H., & Paul, V. J. (2009). Phylogenetic and Chemical Diversity of Three Chemotypes of Bloom-Forming Lyngbya Species (Cyanobacteria : Oscillatoriales) from Reefs of Southeastern Florida. Applied and Environmental Microbiology, 75(9), 2879–2888.
–
Silva A., Souza, Aires. Dutra, I. (2014). Ocorrência de algas cianofíticas em água de dessedentação de bovinos criados extensivamente. Pesquisa Veterinária Brasileira. 34. 415-420.
–
United States Department of Agriculture. (2020). 9 CFR Part 91 Program Handbook.
–
Wilson, A. E., Chislock, M. F., Yang, Z., Barros, M. U. G., & Roberts, J. F. (2018). Pond bank access as an approach for managing toxic cyanobacteria in beef cattle pasture drinking water ponds. Environmental Monitoring and Assessment, 190(4).
–
Willms, W. D., Kenzie, O. R., McAllister, T. A., Colwell, D., Veira, D., Wilmshurst, J. F., Entz, T., & Olson, M. E. (2002). Effects of Water Quality on Cattle Performance. Journal of Range Management, 55(5), 452.
–
Yang, X.-e., Wu, X., Hao, H.-l., & He, Z.-l. (2008). Mechanisms and assessment of water eutrophication. Journal of Zhejiang University Science B, 9(3), 197–209.
- Leptospirosis in Cattle:Why You Should Know More About It? - July 11, 2025
- Dangers of Toxic Algae Blooms for Cattle: Risks and Prevention Strategies - December 20, 2024
- Gastrointestinal (GI) Parasites of Beef Cow-Calf Operations in Florida - June 14, 2024
