Fertilizer source is one of the four Rs of fertilizer management (Right source, Right place, Right time, and Right placement). Mineral fertilizers are typically synthesized (i.e., nitrogen from the Haber-Bosch process) or mined from mineral rock and they are available to the plant in salt form. Salts are water soluble and will dissociate into their positively and negatively charged components (i.e., ions), much like table salt (NaCl →Na+ and Cl–). As one might imagine, these water-soluble fertilizers are often readily available to plants, but with heavy rainfall events or excess irrigation, they can also escape from the land via run-off or soil leaching.
In comparison, organic-derived fertilizer alternatives are not readily water soluble because most of their nutrients exist in organic forms (molecules that contain carbon). Over time, these molecules can mineralize through physical and chemical interactions with the soil biology. Soil microorganisms will use the released carbon as energy, while some of the mineralized water-soluble nutrients will be released into the soil. Sometimes the carbon:nitrogen ratio (C:N) of an organic material is so high that the microbe population will utilize all mineralized N (immobilize) which leaves little for plant uptake. To avoid N immobilization, materials should have C:N ratios around 30:1 or less. Even immobilized N will eventually be released again by competing microbes as their carbon supply dwindles. This will be quicker under warm, wet conditions (Florida summers). People often mistakenly believe plant essential elements derived from organic fertilizer sources are “better” than nutrients from mineral sources. Plants can take up dissolved nutrients regardless of their origin (organic or mineral), if they are in ionic forms but other factors should be considered.
–

Figure 1. Visual appearance of a typical fertilizer blend compared to organic fertilizer sources: poultry manure, cattle manure, horse manure, and Class AA biosolids. Credit: Cheryl Mackowiak, UF/IFAS
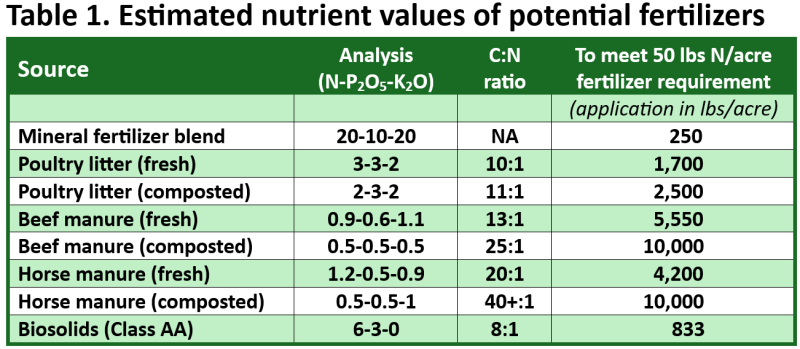
There are several types of organic materials one might consider using as fertilizer. A few of these include poultry litter, cattle manure, horse manure, and biosolids or treated municipal wastewater residuals in Figure 1 (above). Approximate comparisons of one mineral fertilizer blend against organic fertilizer alternatives are given in Table 1 (below).
Manures may contain human pathogens, so a long re-entry period (USDA 90-to-120-day rule) is required before human food crops can be grown on commercial farms using manures. In comparison, composted manures should have gone through the composting process within a temperature range of 131 to 170°F for at least 3 days (if using in-vessel or static-aerated pile) or at least 15 days if windrow composting is used (and the windrow turned at least 5 times). The high heat treatment destroys human pathogens. Composted manures purchased at stores should meet these requirements and are acceptable to use in vegetable gardens. Only Class AA biosolids have been heat-treated to kill human pathogens and are acceptable to apply to vegetable gardens. Class B biosolids have not been heat-treated and therefore are not recommended for use on human food crops.
Nitrogen (N), potash (K2O), and phosphate (P2O5) should be provided on the “fertilizer analysis label” for any material sold as fertilizer in Florida. For example, if you see 20-5-10 on a fertilizer label, the material contains (in order) 20% N, 5% P2O5, and 10% K2O. In comparison, organic derived fertilizer alternatives may (if sold as a fertilizer) or may not (not sold as a fertilizer but has fertilizer value) have an analysis label. Unlike most fertilizer blends (unless specifically requested), fertilizer alternatives contain additional plant essential nutrients, such as sulfur, calcium, magnesium, iron, manganese, boron, zinc, and copper.
Uncoated mineral fertilizers release their nutrients rapidly (within days or weeks), while mineralization of organic fertilizers is relatively slow. They tend to release all their potassium and most of their phosphorus within the first growing season. In comparison, only about 30 to 50% of total N by the end of a summer growing season, depending on the product. Biosolids are somewhat more nutrient rich compared to manures, but these products have similar nutrient release rates. Unlike many manures, biosolids typically contain a negligible amount of potassium (Table 1). The N:P ratio is often lower in organic fertilizers than what a crop requires if product is being applied to supply 100% of plant N fertilizer requirement. This results in excessively high soil P fertility over time, which can interfere with the uptake of other essential nutrients like iron and zinc. That is why applying organic materials based on the soil P requirement and using another N fertilizer source to make up any additional N deficit will help keep your soil fertility in balance and protect the environment from P pollutants. Having your soil analyzed for fertility (soil test report) will help maintain soil fertility balance and likely save you fertilizer dollars over time. The UF/IFAS extension soil testing lab (ESTL), in Gainesville, Florida accepts samples for a relatively low fee (contact your county extension office for details) or there are several commercial labs throughout the southeast providing similar services.
Composted manures are somewhat lower in nutrients than fresh manures and they have greater C:N ratios. Additionally, horse manures may immobilize N for a time, due to high C:N ratios. Often, composted cattle and horse manures are used as soil amendments (build soil health when worked into the soil) more than as a fertilizer source. Labs can also test soil organic matter (SOM). Values of 2% or greater often reflect relatively healthy soil under Florida’s challenging conditions.
Rather than an analysis label, many manure products sold outside stores will list pounds of a given nutrient per ton of material (and provide the percent moisture it was tested at). If the bulk material you receive seems drier than when the analysis was taken, the nutrients will be more concentrated (requiring less per acre application rates), while wetter products (like what sometimes happen with poultry litters) will have their nutrient composition diluted, thereby requiring greater application rates. Additionally, if you smell ammonia coming off any fertilizer product, that is nitrogen being lost from the fertilizer to the atmosphere. If you need to delay application, store it dry (cover with a tarp, if outdoors). Also monitor stockpiled manure and immature composted manure for heat. Smoldering or exploding manure can ruin your day!
- Fertilizer Source Matters! Comparison of Alternatives for Nutrient Content - January 16, 2026
- Grower Survey Insights on Cotton Nitrogen Management in Florida’s Panhandle - July 18, 2025
- Florida Soils are an Indispensable Natural Resource - January 10, 2025