João H.J. Bittar, DVM, Ph.D., Ameer A. Megahed, DVM, Ph.D., Ahmadreza Mirzaei, DVM, PhD., Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida
What is BLV?
Bovine Leukosis is a lifelong infection caused by the bovine leukemia virus (BLV), a pathogen that infects cattle white blood cells (lymphocytes) in general by inserting its genomic material into the host genome. Technically it is named Enzootic Bovine Leukosis (EBL). The first descriptions of EBL were made about 150 years ago in Germany, and reports of this disease became common after World War II. Enzootic bovine leukosis is a contagious chronic autoimmune disorder (lymphoproliferative) of cattle, characterized by a continuous increase of white blood cells (persistent lymphocytosis) that can end with B-cell lymphoma, also known as Hodgkin lymphoma or non-Hodgkin lymphoma, especially in immune-compromised animals. In this case, a type of blood cell called B-cells becomes abnormal and can no longer fight off infection. Enzootic bovine leukosis is widely distributed, and it was listed by the World Organization for Animal Health (OIE) as a disease that could significantly impact international trade.
Bovine leukemia virus infects cattle, and it can present in one of three forms: 1) asymptomatic or aleukemic (cows do not display outward signs of disease), 2) persistent lymphocytosis (cows continuously show an increase in lymphocyte counts), and 3) leukemia or lymphoma/lymphosarcoma (leukemia is a type of tumor located in the bone marrow and blood, whereas lymphoma [or lymphosarcoma] is a tumor located in the lymph nodes). Approximately 60 to 65% of infected cattle are aleukemic, 30 to 40% have persistent lymphocytosis, and less than 5% develop malignant lymphosarcoma, which is considered the most common neoplastic disease identified in slaughtered cattle in the United States. Older multiparous cows (≥ 5 calves) are most likely to display clinical signs, while cows less than 2-years-old are present most frequently as asymptomatic.
–
Impact of BLV
In both the dairy and beef industries, BLV infection results in high economic losses due to reduced milk quantity and quality, lower fertility rates, increased heifer replacement costs, and veterinarian costs (consulting, medical care of the sick animals, and diagnostics). In addition to the inability to export cattle, semen, and embryos to countries that maintain BLV control programs, such as the European Union. The economic losses of lymphosarcoma were estimated to be $412 per case, and the yearly direct losses in the dairy industry associated with clinical BLV infections are more than $500 million. The average annual cost in a 50% prevalence herd was nearly $6,400 per 100 milking cows.
The USDA National Animal Health Monitoring System (USDA-NAHMS) conducted two survey studies on US dairies in 1996 and 2007 and showed a high prevalence of BLV in dairy herds (83% of operations overall had bulk-tank tested positive for BLV in the USDA-NAHMS 2007 survey). Further, 7.5% of dairy operations that submitted test samples for BLV had a positive laboratory BLV test in the previous year (USDA-NAHMS survey of 2007). Another study using data from these two NAHMS surveys estimated that herds with BLV test-positive cows produced annually 481 lbs. less milk per cow than herds that were BLV-negative. Herds were determined to be negative based on the absence of serum antibodies to BLV in agar-gel immunodiffusion test, in which only 114 herds fit this criterion out of 976 totals. The mean annual value of production per cow decreased by $1.28 for each 1% increase in herd BLV prevalence at a milk price of $0.13 per lb. The longer a cow was in persistent lymphocytosis showed a progressive milk loss. Intriguingly, BLV-positive cows showed significantly less milk production only in the early and middle stages of lactation than BLV-negative cows. Additionally, a higher somatic cell score in BLV-positive cows was also reported during the early and middle stages of lactation. It has been reported that the cattle infected with BLV showed impaired immune response to J5 Escherichia coli bacterin and foot-and-mouth disease vaccines.
–
How is BLV Transmitted?
Typically, BLV is not free in the peripheral blood, but the virus is integrated with the lymphocytes of different body fluids, mostly milk and blood. Therefore, BLV is primarily transmitted through the blood of infected cattle but to a lesser extent, may transmit through milk, saliva, and semen. Thus, the management practices that lead to direct exposure to infected blood, such as the use of common needles, blood-contaminated syringes, rectal sleeves, tattooing, and ear tagging, may increase the transmission of BLV infection. It is believed that BLV-infected cattle with persistent lymphocytosis can harbor more virus per lymphocyte in circulating blood and more readily transmit BLV.
The incubation period is the time of exposure and infection of a pathogen to the first appearance of associated clinical signs. In regards to BLV, the incubation period ranges from 5 to 10 years, with an average of 7 years. The specific clinical signs include lymphocytosis (increased lymphocyte counts in the peripheral blood), lymphadenopathy (peripheral and/or internal lymph node enlargement [Figures 1 and 2]) with or without brisket edema, jugular vein distention, bloat, rear limb weakness or paralysis, exophthalmia (protruding eyeball), and melena (feces with the presence of digested blood). Other specific clinical signs that are associated with other general clinical signs are fever, loss of appetite, dyspnea (labored breathing), tachycardia (increased heart rate), decreased milk production, and weight loss.

Figure 1. Dairy cow infected with BLV with enlarged parotid, submandibular, and prescapular lymph node. Credit: VeeproHolland
–
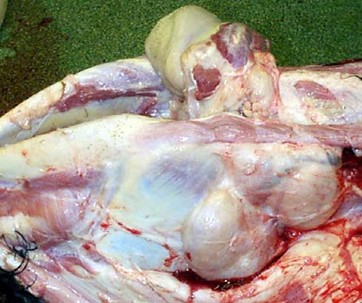
Figure 2. Enlarged submandibular lymph nodes (10 to 30 times larger than the normal size) due to BLV infection . Credit: Scott, 2011.
Testing fro BLV
Typically, diagnosing the BLV infection includes direct detection of the virus in body fluids and/or indirect detection of serum BLV antibodies, also known as serology testing. A serology test looks for antibodies in the blood that fight the virus, in this case, BLV. Direct detection of BLV antigen is performed through two methods: 1) virus isolation and propagation, which is not routinely used because of low viral gene expression and difficulties of virus propagation; 2) direct detection of BLV virus using polymerase chain reaction (PCR), also used with limited success because of its highly mutable RNA genome. Additionally, traditional PCR is not developed for detecting BLV in milk, which is the source of many transgenic proteins. However, the real-time and nested PCR can provide a valuable method for early detection of BLV and confirming BLV infection from blood and milk samples.
Commercial ELISA kits are the most popular serological test for detecting BLV infection in cattle because they are rapid, inexpensive, and easy to interpret. However, the antibodies against BLV may not be produced until 14 weeks after infection, when the infected animals are viremic and transmit the BLV to other animals. Additionally, the serological tests cannot be differentiated between active and passive immunity. For instance, bulk tank milk ELISA is frequently applied for surveillance of BLV, where the infected animal remains infected for life and generates a continuous antibody response which adds credibility to serological tests.
–
BLV Prevention
Currently, there are no treatments available for animals infected with BLV. Therefore, prevention and control of infection are the only effective methods to stop the spread of BLV between animals. To date, there is no available vaccine against BLV in cattle, so the only way to prevent the spread of BLV among cattle is to control the transmission between infected animals and susceptible cattle. Control of BLV infection at the national level is usually performed through one or more of the following three approaches: 1) test and segregation, 2) test and slaughter, 3) test and manage, and apply effective biosafety and management measures to minimize or prevent BLV exposure. The selection and success of each strategy are mainly dependent on having a reliable estimate of the within-herd prevalence. Currently, in the United States, the test-and-removal strategy is voluntary.
It is important to note that the first and second approaches will not eradicate BLV from the herd without applying restricted hygienic-management procedures to reduce exposure to BLV, such as avoiding the sharing of needles, changing rectal sleeves/gloves between each palpation, and thoroughly disinfecting tools and equipment that can share blood (tattooing pliers, ear tag pliers, IV tubing, dehorning instruments, castration knives, hoof trimming knives, hormone implant devices, and similar instruments), and use of insecticide to lower the blood-feeding insects’ population. It has been suggested that at least 219 yards boundary exists between the primary herd and the BLV-infected herd to prevent transmission.
US cattle herds are still susceptible to BLV infection, and the associated losses have a negative impact on cattle production and performance. These effects are even more remarkable when the lymphoma-leukemia form is present in the herd due to carcass condemnation at the slaughterhouse in cattle with clinical signs. It is relevant to note that most research related to the determination of seroprevalence of BLV in the US was conducted on dairy cattle. Similar seroprevalence studies should be conducted on beef cattle herds. Therefore, education and research aimed at BLV control and prevention strategies are of value and warranted for the US cattle industry.
If you suspect having cases of cattle with bovine leukemia virus, contact your veterinarian to get adequate help with diagnosis and future management practices to reduce further losses related to this disease.
